Did you know that some stones that look completely ordinary in daylight can glow under UV light? These special rocks are called fluorescent minerals.
Fluorescence is like a hidden superpower. When these minerals are exposed to invisible ultraviolet (UV) light, they absorb that energy and glow with bright colors.
This effect was first noticed in 1824 by Friedrich Mohs while studying fluorite. The word “fluorescence” comes from this discovery.
🌈 Why Do Some Rocks Glow?
Most minerals don’t glow at all — only a small number have the right ingredients or tiny flaws that allow them to fluoresce.
Here’s what makes the glowing magic happen:
• Special impurities called “activators.”
Some minerals contain trace amounts of elements such as manganese, uranium, lead, and rare-earth elements. These tiny impurities absorb UV light and release it as visible light, creating the glow we see.
• Crystal structure and defects.
Even without many activators, the way a mineral’s atoms are arranged — or small defects in that arrangement — can allow fluorescence to occur.
• Using UV Light
UV light comes in different wavelengths. Some minerals glow only under short-wave UV, while others glow only under long-wave UV. Even two pieces of the same mineral don’t always act the same — one might glow under UV light while another stays completely dark.
Because all these factors vary so much, mineral fluorescence can be unpredictable — which is exactly why finding glowing rocks feels like a treasure hunt!
LBJD Black Light UV Flashlight (365nm)
- Why I Like It: This light is affordable, easy to use, rechargeable, and perfect for beginners.
- It's small enough to carry in your pocket and powerful enough to light up rocks like yooperlites or calcite.
- Best For: General rockhounding, casual, and home use.
If you make a purchase via our links, Northwest Rockhounding earns a commission at no additional cost to you
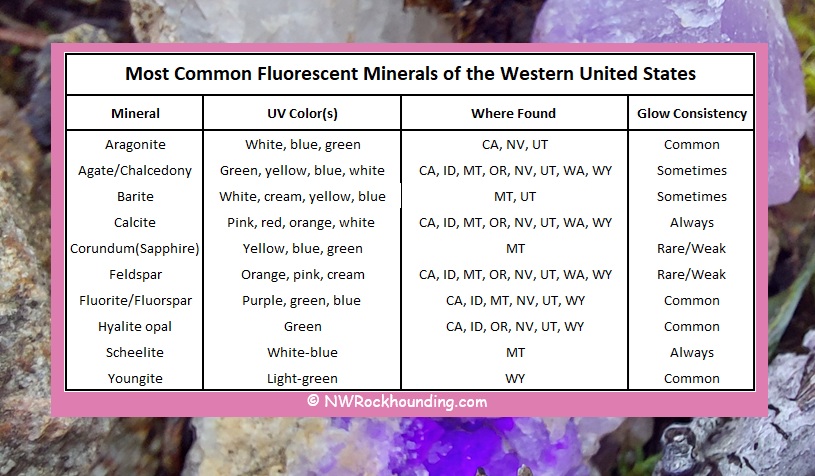
Where to Find Fluorescent Minerals in the Western United States
Many fluorescent minerals occur across the Western United States. Minerals such as calcite, fluorescent chalcedony, and feldspar are widely distributed and can be found throughout all the western states.
California hosts hyalite in Northeastern California, fluorspar, scheelite, aragonite, and opal at sites such as the Gem Hill, and the Southern California tungsten districts.
Idaho offers hyalite opal at Fir Grove Mountain and fluorite in the Challis area.
Montana is known for fluorite from Snowbird Mine and barite from the Butte and Great Falls regions. Missouri River and Gem Mountain sapphires (gem-quality corundum) show weak to moderate fluorescene.
In Oregon, hyalite opal, fluorescent feldspar and thundereggs can be found near Central Oregon volcanic fields and around Lakeview.
Nevada is one of the richest states for fluorescent minerals, with classic specimens such as aragonite, fluorite, and hyalite opal found in places like Tonopah and Broken Hills Mining Districts, and Virgin Valley opal fields.
Utah provides excellent collecting for fluorescent fluorite, hyalite opal, and barite at locations such as the Dugway Geode Beds, Topaz Mountain, and the Confusion Range.
Wyoming is best known for youngite and opal in areas such as the Youngite collecting area near Guernsey.
UV Lights in the Field
Using a UV light in the field helps you discover things you’d never notice in normal daylight.
When UV light shines on certain rocks, they glow in bright colors such as green, pink, orange, or blue. It makes rockhounding more exciting and helps you spot unique pieces, whether you’re out during the day or at night.
A UV light can reveal minerals such as calcite, hyalite opal, and fluorite—rocks that are easy to overlook with just your eyes.
Fluorescence as Basic Mineral Identification
Fluorescence can be a helpful clue when identifying minerals, but it isn’t always reliable. Even pieces of the same mineral from the same location may behave differently — some will fluoresce strongly, others weakly, and some not at all.
Because of this inconsistency, fluorescence should be used as just one part of the identification process, alongside other tests and observations.
You May Also Like









